VALENCE
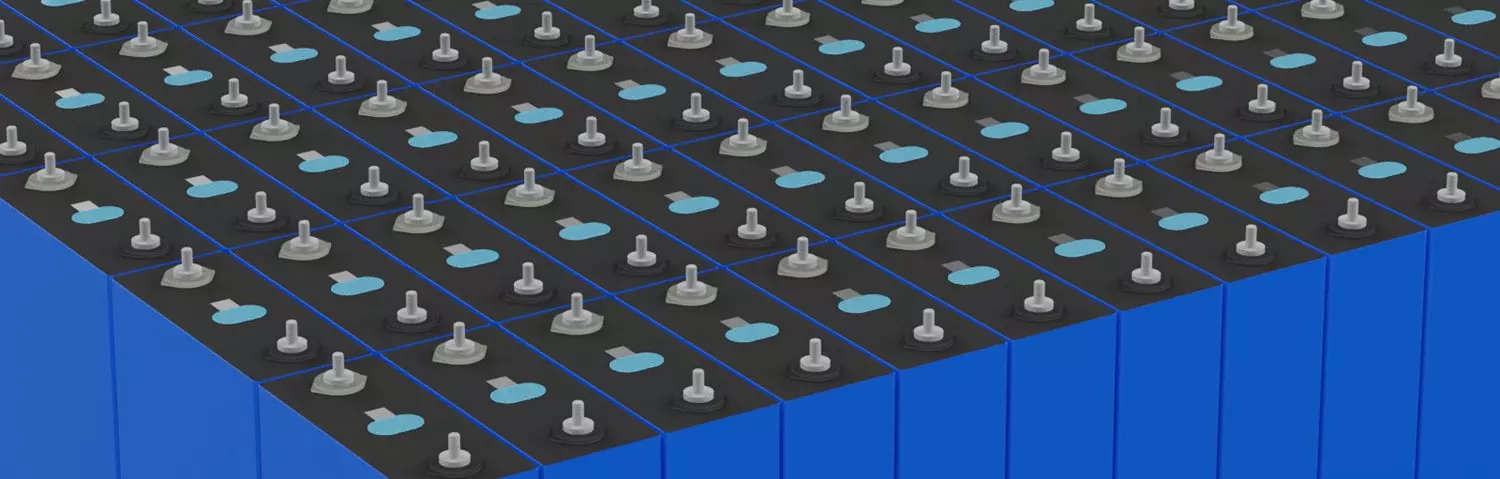
LiFePO4-Zellen
LiFePO4-Zellen, Energiespeicherbatterie. One Stop Services.
-
Lösung Lithium Eisenphosphat -
Lösung Lithium Ionen Akku
Valenz - The Fundamental Concept in Chemistry In the realm of chemistry, Valenz is a crucial concept that defines the number of electrons in an atom that participate in forming chemical bonds with other atoms. This crucial aspect of atomic structure plays a vital role in determining the properties and behavior of elements, ultimately influencing their reactivity and chemical interactions. The Valenz of an atom is equivalent to the number of electrons it has in its outermost energy level or shell. Atomic orbitals, which are the regions around the nucleus where electrons are likely to be found, hold the key to understanding the concept of Valenz. Electrons in these orbitals exhibit unique properties, such as the ability to participate in chemical bonding, based on the number of electrons present in each orbital. Understanding the Valenz of atoms is vital for predicting the properties and behavior of elements, including their reactivity and propensity to form chemical compounds. The concept of Valenz is closely tied to the idea of oxidation states, which describe the number of electrons gained or lost by an atom during a chemical reaction. In chemical reactions, changes in Valenz occur when atoms gain or lose electrons, resulting in the formation of ions with unique properties. Understanding these changes allows chemists to predict the reactions that will occur when elements combine, ultimately enabling the design and development of complex chemical systems. For chemists, understanding the Valenz of atoms is a fundamental aspect of their work. By grasping the concept of Valenz, chemists can develop a deeper understanding of the intricate processes that govern chemical reactions, ultimately advancing the development of new materials, fuels, and technologies. Whether in the laboratory or the real world, a solid grasp of Valenz is essential for unlocking the secrets of matter and unlocking its potential.